
Close

Accurate fountain (dampening) solution concentration control is essential for consistent, high-quality results in lithography. Low concentration can cause drying on the non-image area of the plate resulting in tinting, scumming, blanket piling, etc. High concentrations, on the other hand, bring about over-emulsification of the ink. This results in weakening of color strength and changes in ink rheology (body and flow properties). Correct concentration will allow the non-image areas of the plate to be appropriately wetted.
Traditionally, pH was the test relied on to determine fountain solution concentration. Today, however, conductivity testing is recognized as a much more accurate method. Many modern dampening solutions are pH stabilized (or buffered), so only small changes in pH are seen even when dramatic changes occur in solution strength. Conductivity measurement is a fast and easy test which is more indicative of fountain solution concentration than pH. This is true for all neutral, alkaline, and many acid type solutions.
pH is still important, however, with unbuffered acid fountain solutions. Checking both conductivity and pH can provide valuable information. Acid fountain solution is a mixture of gum arabic, wetting agents, salts, acids, buffers, etc. Conductivity will tell you if the proper amount of most ingredients are present, but pH is necessary to check acid concentrations. pH will also determine how effective one ingredient, gum arabic, will be.
What is conductivity? Conductivity is the measurement of a solution’s ability to conduct an electrical current. It is usually expressed in microsiemens (micromhos). Absolutely pure water is actually a poor electrical conductor. It is the substances dissolved in water which determine how conductive the solution will be. Therefore, conductivity is an excellent indicator of solution strength.
1. Test and write down the conductivity of the water used to prepare the solution.
2. Mix the fountain solution concentrate with the water, using the manufacturer’s recommendations or as experience dictates.
3. Measure the conductivity of the mixed solution.
4. Subtract the water conductivity value obtained in step 1. This is necessary because tap water quality can change from day to day.
The resulting number is an accurate indicator of fountain solution strength. Caution: because alcohol will lower a solution’s conductivity, always test solution conductivity before and after the addition of alcohol.
Determining the best concentration of fountain solution is mostly “trial and error.” It can be very useful to make a graph, recording readings for every one-half or one ounce of concentrate added to a gallon of water. Record readings on a graph with the vertical axis representing conductivity values and the horizontal axis representing ounces/gallon. Such a graph will help “fine tune” your system during future press runs.
For “on the spot” fountain solution tests, Myron L® handheld instruments are fast, accurate, and reliable. Measurements are made in seconds simply by pouring a small sample of solution into the instrument cell cup and pressing a button. Automatic temperature compensated accuracy and famous Myron L® reliability have made our instruments popular in pressrooms worldwide.
Even though pH usually is not the best method to check the concentration of fountain solution, it is still very important and must be checked regularly. The pH of acid dampening solution affects sensitivity, plate-life, ink-drying, etc. Also, pH can change during a run if the paper has a high acid or alkaline content. pH, therefore, must be maintained at the proper level for good printing.
A convenient and accurate way to test pH (as well as temperature) is Myron L® Company’s waterproof Ultrameter II™ Model 6PFCE. The 6PFCE has a 100 reading memory to store test results on site. The 6PFCE also measures conductivity. All electrodes are contained in the cell cup for protection. Model M6/PH also measures pH and conductivity.
For continuous monitoring and controlling of fountain solution concentration, Myron L® offers a variety of in-line monitor/controllers.
The 900 Series Multi-Parameter Monitor/Controller™ models simultaneously monitor and/or control multiple inputs and outputs. They are autoranging with 3 automatic temperature compensation standards for greatest accuracy (KCl, NaCl, and 442 Natural Water™). Input parameters include conductivity, resistivity, TDS, salinity, pH, ORP, temperature, mV input, flow, % Rejection and a 4-20 mA multi-parameter input. The 900M-3C has 3 alarm/ control relays and 2 remote alarm outputs (configurable), as well as a 0-10 VDC recorder output, 4-20 mA output, and an RS-485 serial output.
The 720 and 750 Series II Monitor/controller models are available for individual parameters.
All Myron L® controller models contain heavy-duty relay(s) with adjustable set point(s) which can be used to activate alarms, valves, feedpumps, etc. Dual set points are quite popular in pressrooms as they allow a “safe zone” for controlling fountain solution concentration. A variety of options and outputs are available to cost-effectively tailor the monitor/controller to your particular application.
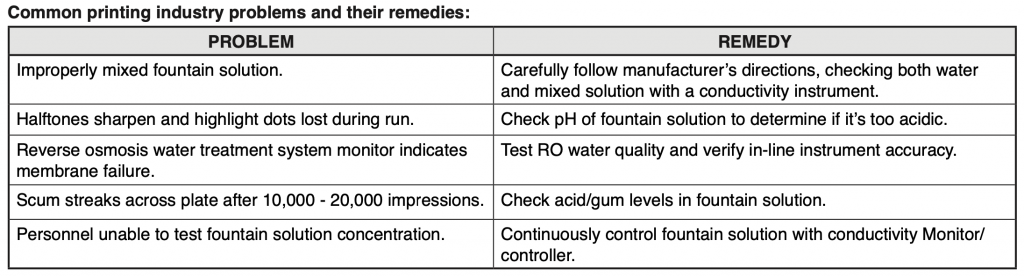
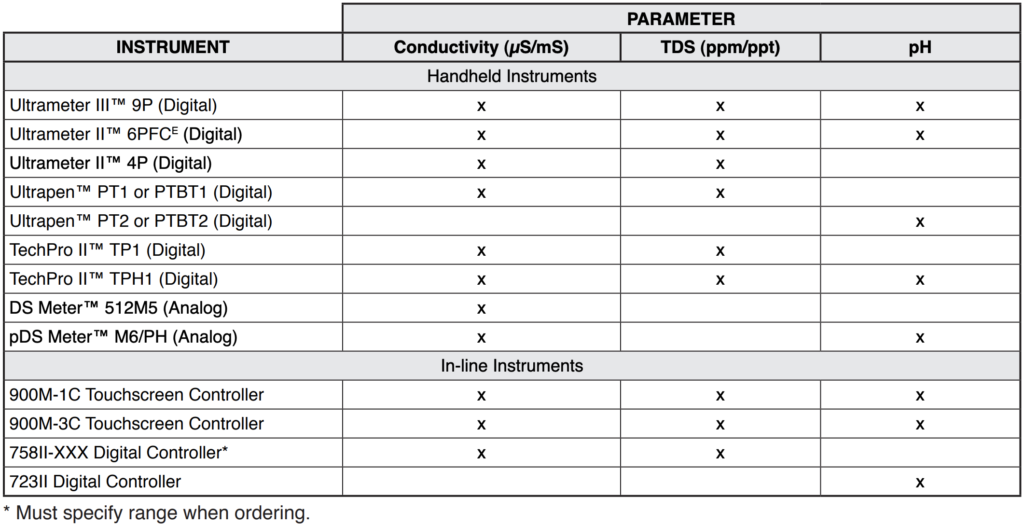
The following table briefly covers some of the Myron L® Company instruments recommended for the printing industry. For further details, please contact your local distributor, refer to Myron L® data sheets, visit our website (www.myronl.com), or contact us by phone, fax, or email ([email protected]).
The Ultrameter II™ 6PFCE, DS Meter™ 512M5 and pDS Meter™ M6/PH are available with the useful LITHO-KIT™. This accessory includes a foam-lined, rugged all-plastic carry case with calibrating solutions and buffers. In addition, a syringe to simplify drawing samples and a thermometer for testing fountain solution temperature are also included.
